Eureco-Pharma keeps its employees on track with Q-Learning
“ManualMaster Trevally works excellently for recording and managing documents,” says Wandena Lalkoe, Quality Assurance Officer at Eureco-Pharma. “Our web forms, created with the WebForms module, are ideal for initiating our processes. With Q-Learning, we train our people and check whether everyone’s knowledge is up to date, ensuring they can do their work correctly.”
Overviews the organization relies on
“The application has made my tasks as QA Officer—such as setting up, managing, and monitoring the quality system, processes, and activities—much easier. It creates overviews we can use to guide the organization. And the best part is that I, as a QA Officer, can continue to develop myself with ManualMaster Trevally. The ManualMaster Academy helps me do that.”
Core values up-to-date in Trevally
Eureco-Pharma has been active for over 25 years in the field of pharmaceutical care in the Netherlands. Their goal is affordable and accessible pharmaceutical care for every patient. Every day, a team of motivated and driven people works to align the supply and demand of medicines within the European market.
Eureco-Pharma operates according to Good Distribution Practice (GDP) and Good Manufacturing Practice (GMP) guidelines to guarantee the quality and safety of medicines. Improving quality standards is a continuous process at Eureco-Pharma, focusing on reliability and expertise. These core values are kept up to date using the ManualMaster Trevally modules Document Management, WebForms, and Q-Learning.

Wandena Lalkoe
About Eureco-Pharma Transport Service
| Main activity | Import and distribution of existing pharmaceutical products |
| Using Trevally since | 2013 |
| Uses Trevally for | Document management – Web forms – Q-Learning |
Document management provides a solid foundation
An important part of quality management at Eureco-Pharma is safeguarding the supply chain. Eureco-Pharma regularly performs risk analyses, inspections, and audits on-site in the supply chain.
“ManualMaster Trevally’s Document Management provides a solid foundation for this and includes our procedures, work instructions, deviations, complaints, CAPA management, and Change Control,” Wandena explains.
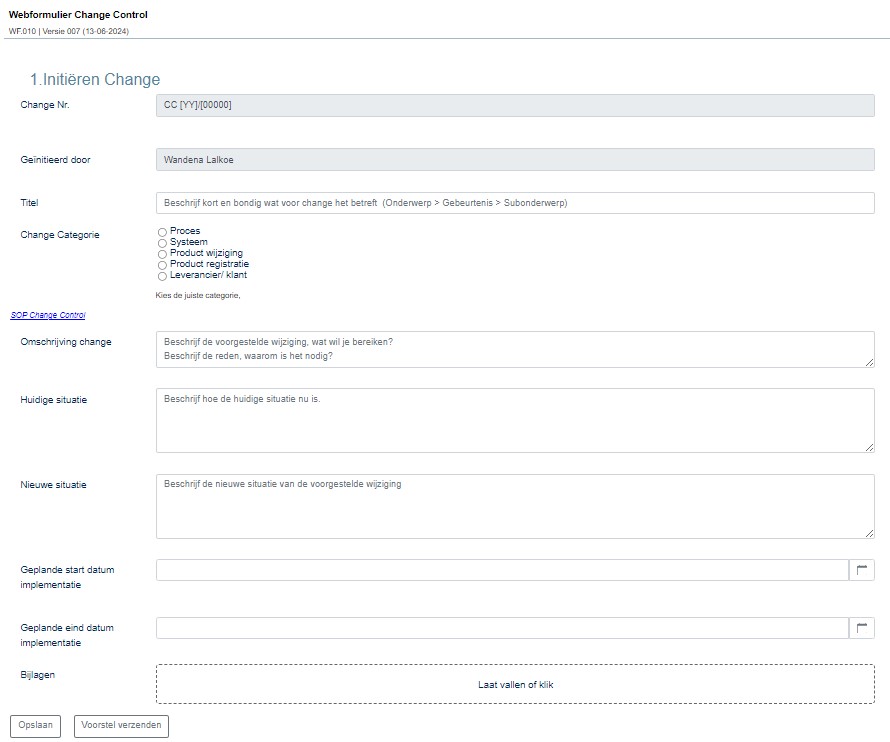
“With our Change Control System, also known as Management of Change (MoC), we evaluate all changes that could affect production and quality. The use of the WebForms module, which allows us to build web forms, plays a crucial role here.”
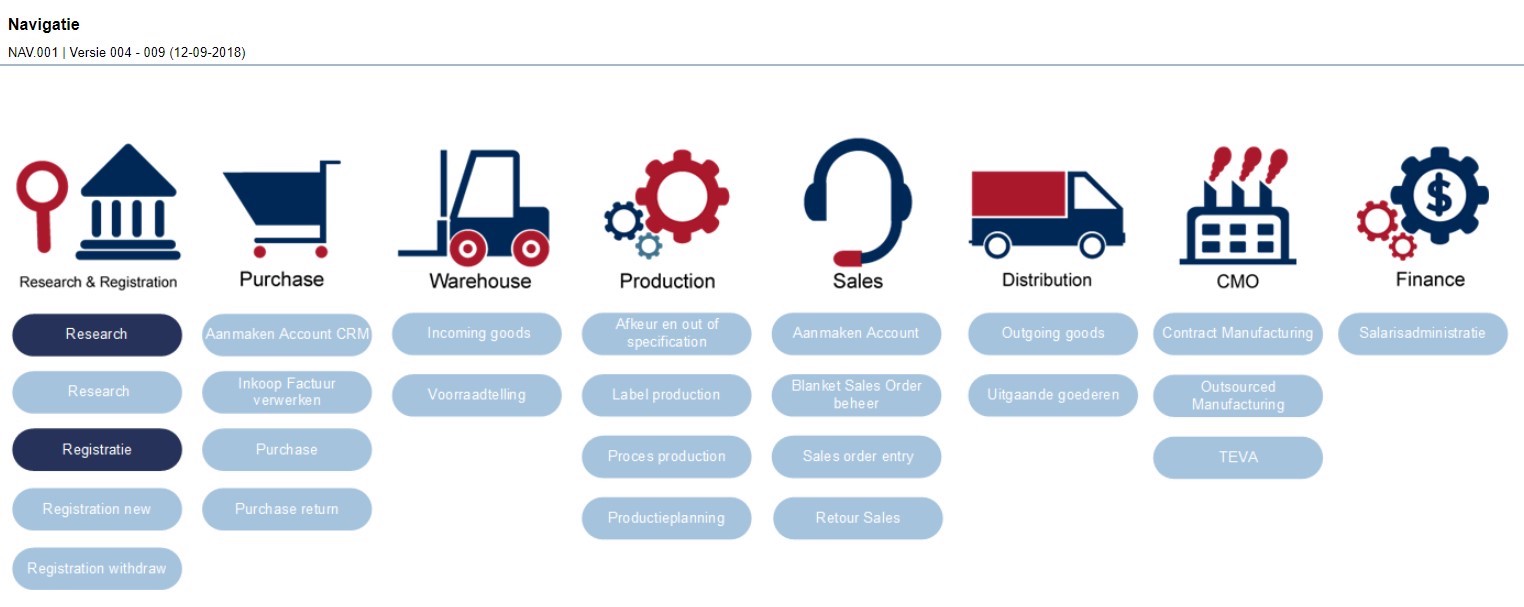
Processes and procedures in flowcharts
All processes and the procedure scan at Eureco-Pharma are described in ManualMaster Trevally and will soon be visualized in flowcharts created with the software.
Wandena: “The GDP and GMP standards included in these documents are linked to the work instructions for our employees. When logging into the system, everyone sees the same dashboard, and depending on their role, they can navigate through the quality system.”
ManualMaster Trevally can show how often—and by whom—the system is used. At Eureco-Pharma, six editors are authorized to make document changes, and the application automatically initiates the validation process.
Testing knowledge with Q-Learning
With Q-Learning, everyone is kept up to date on the latest developments regarding standards and guidelines, and their knowledge can be tested.
Using this module, Wandena has an overview of which training has been assigned to whom and how far trainees have progressed. “For instance, our training courses are linked to the GDP and GMP guidelines we have to comply with. These are repeated annually, and with Q-Learning we can easily monitor the progress of this process.”
Q-Learning closely monitors training
“That’s why we make intensive use of Q-Learning. We’re very satisfied with the module. Previously, training did not go as we wanted. We couldn’t monitor it, didn’t know who was following which training course, and had no idea whether someone had started their training or how far along they were. With Q-Learning, we can track all of that closely.
We’ve kept the training for our colleagues as simple as possible. First, they receive theoretical knowledge through Q-Learning, then they are asked questions about the material. Everyone can follow the training well. In practice, training may sometimes need to be adapted to the level of the participants. But that’s also easy to manage.”
Web forms support the quality system
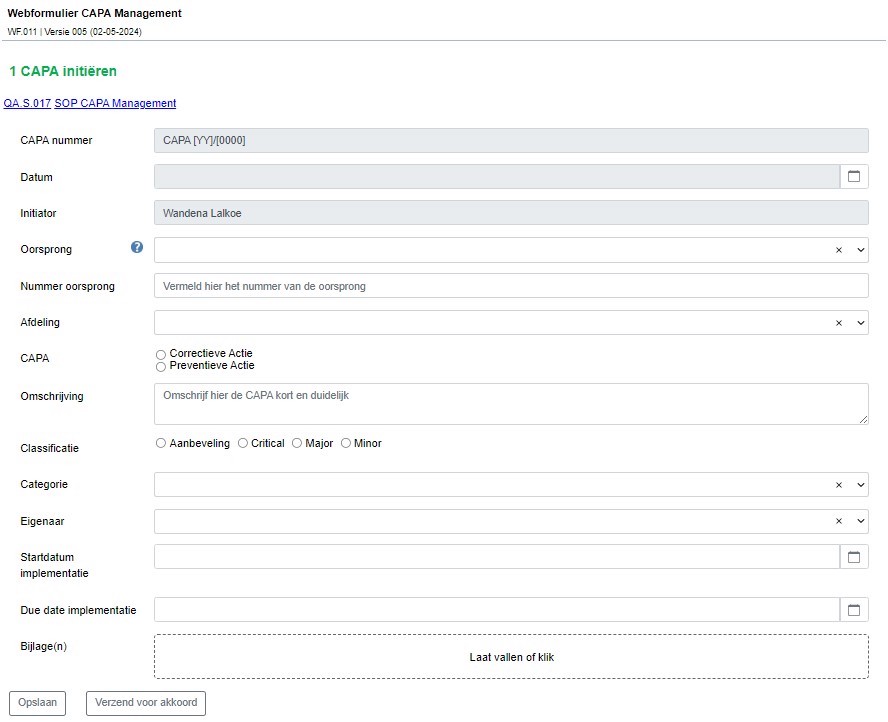
The web forms that Wandena and her colleagues create with the WebForms module are used for visitor registration, recording and following up on deviations and complaints, Change Control forms, and CAPA Management. More forms are in development to support Eureco-Pharma’s quality system.
Action forms with assessments are linked to the Change Control forms, which are automatically sent to the relevant colleagues. ManualMaster Trevally’s web forms can be set up so that it’s immediately clear who made what changes and where in a form. This automatically creates an audit trail.
Following up on workflows with web forms
Wandena: “The web forms help us enormously in recording processes, particularly those involving multiple departments and people. Suppose a deviation is detected in production. Then it must be picked up by QA, and ultimately the production manager has to approve a report. This workflow is made very simple and clear with web forms.”
“Creating web forms does require you to have a good overview of the process in question. You have to stay focused and enjoy building web forms. Personally, I didn’t need much explanation. I really enjoy working with ManualMaster Trevally and get a lot of satisfaction out of it.”