‘Our employees can access immediate information about the process, the risks and what is expected of them’
HP Valves – a producer of high-pressure valves and accessories used in power plants worldwide – is a major supplier to contractors and original equipment manufacturers such as Siemens, NEM Energy, Mitsubishi-Hitachi Power Systems and General Electric.
The company has to comply with quality requirements and standards under ISO 9001, ISO 14001 and PED (the Pressure Equipment Directive). HP Valves uses ManualMaster for process, document and risk management and the registration of nonconformities such as accidents and dangerous situations. QESH Manager Jeroen Nieuwenhuis: ‘Safety rules, working agreements and the knowledge stored in the heads of employees are recorded in ManualMaster. We want to prevent the loss of valuable knowledge – ‘lessons learned’ – when a colleague leaves the company. Typically, this relates to agreements about what has been decided and why and to whom those agreements apply.’
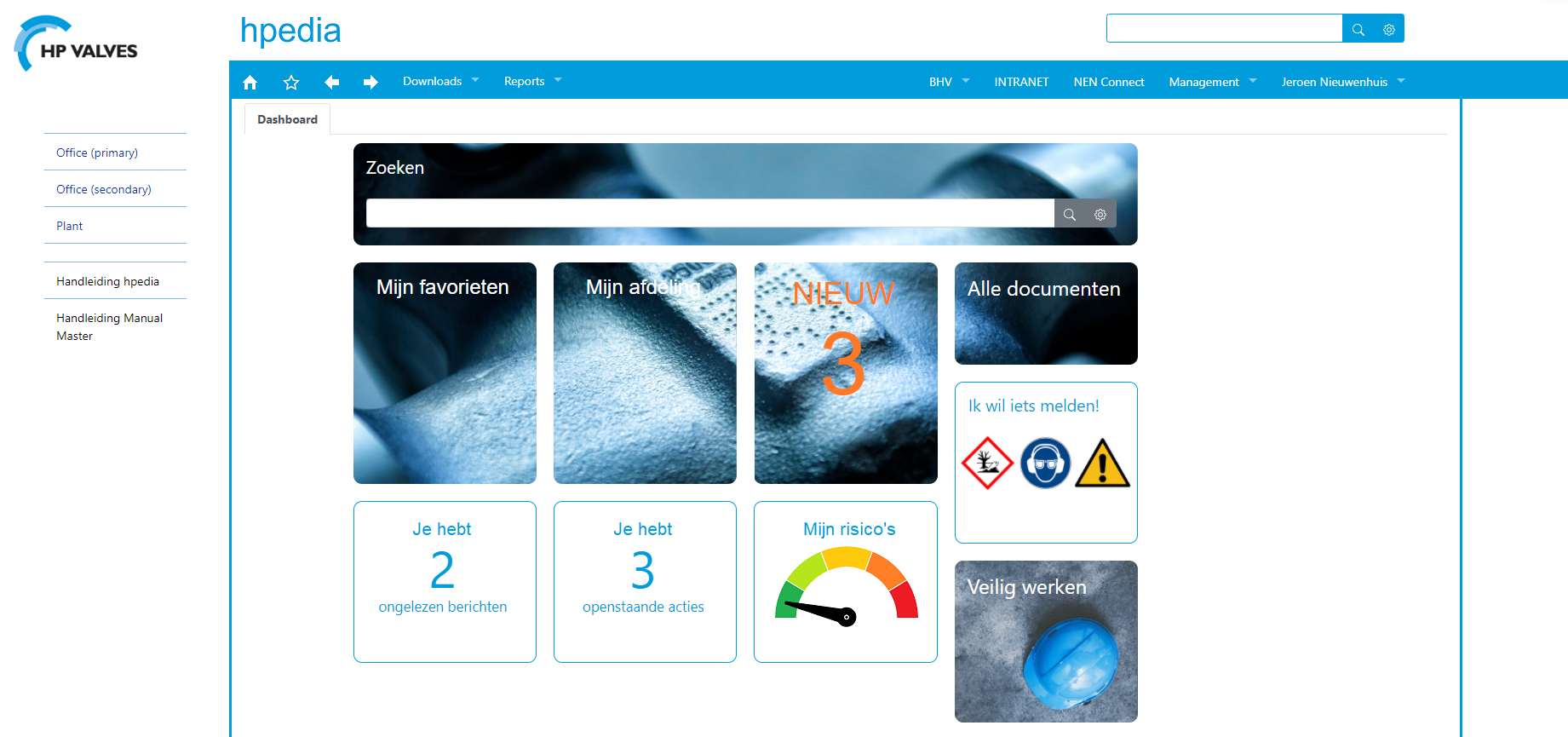
All agreements documented and safely stored
‘We discuss all kinds of topics and take decisions on them. A common problem is that people often can’t remember those thought processes five years later. Past solutions are forgotten and we have to reinvent the wheel. ManualMaster prevents this. All agreements, even the most basic ones, are documented and stored in the ‘encyclopaedia’ in our quality system. We call it Hpedia and all employees can access it.’
(see fig. 1)
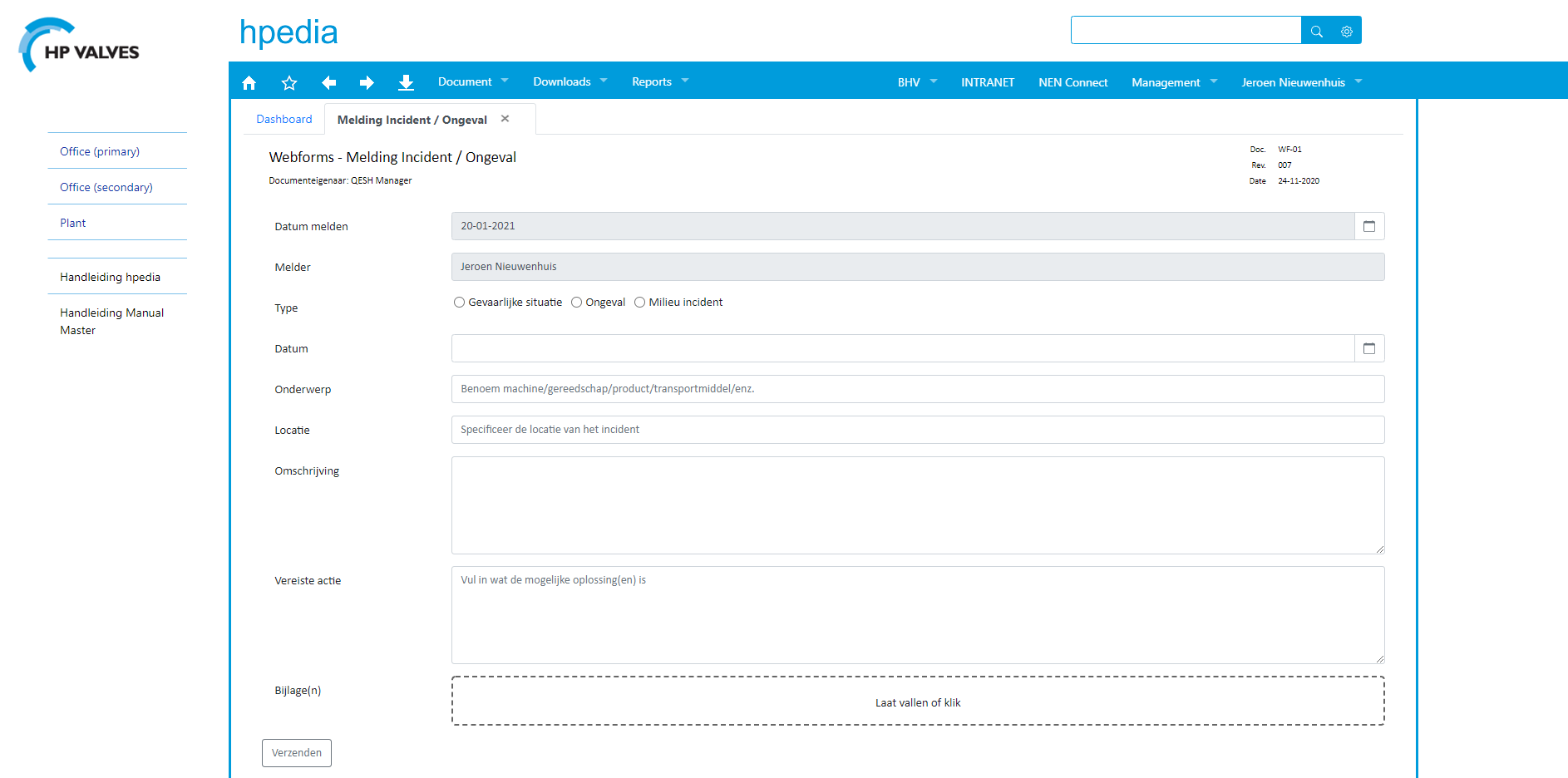
Reporting nonconformities & risk management
HP Valves is now proficient at creating web forms (using the WebForms module in ManualMaster) and linking risks to operating processes (the Risk Management functionality in ManualMaster).
We have defined measures for remedying the situation and preventing re-occurrence and linked them to each risk
Jeroen: ‘We use Risk Management primarily to focus on process risks; i.e. risks to personal safety that arise during the production process. After the probability x impact has been recorded in the web form, each report of an accident or dangerous situation ends with the following question: ‘add to risk analysis?’ Doing so creates a clear record of what you have done to prevent re-occurrence of the situation and correct the problem.’
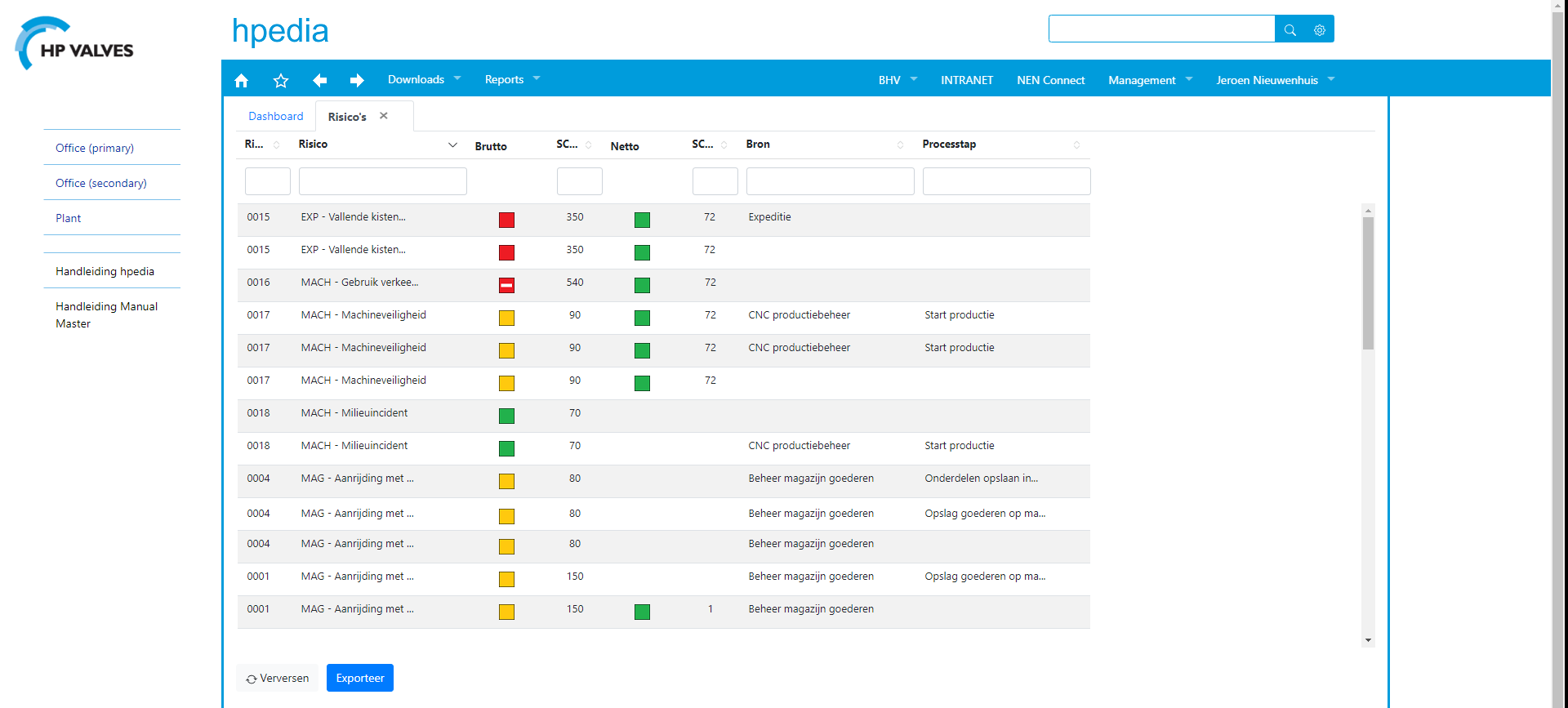
Risk overview and the effect of measures
‘‘Simple presentation of risk registration is a must’, according to the QESH manager. ‘You have to be able to see at a glance how significant a risk is, what measures have been taken and what needs to be done.’
(see fig. 2a and 2b)
‘We have defined measures for remedying the situation and preventing re-occurrence and linked them to each risk. The effectiveness of those measures can be assessed (subsequently) based on accident registration statistics. In addition, we also focus on (preventive) communication to mitigate the more significant risks. The Risk Management function has been set up in a way that guarantees effective coverage of our risks.’
Risks and measures linked to processes
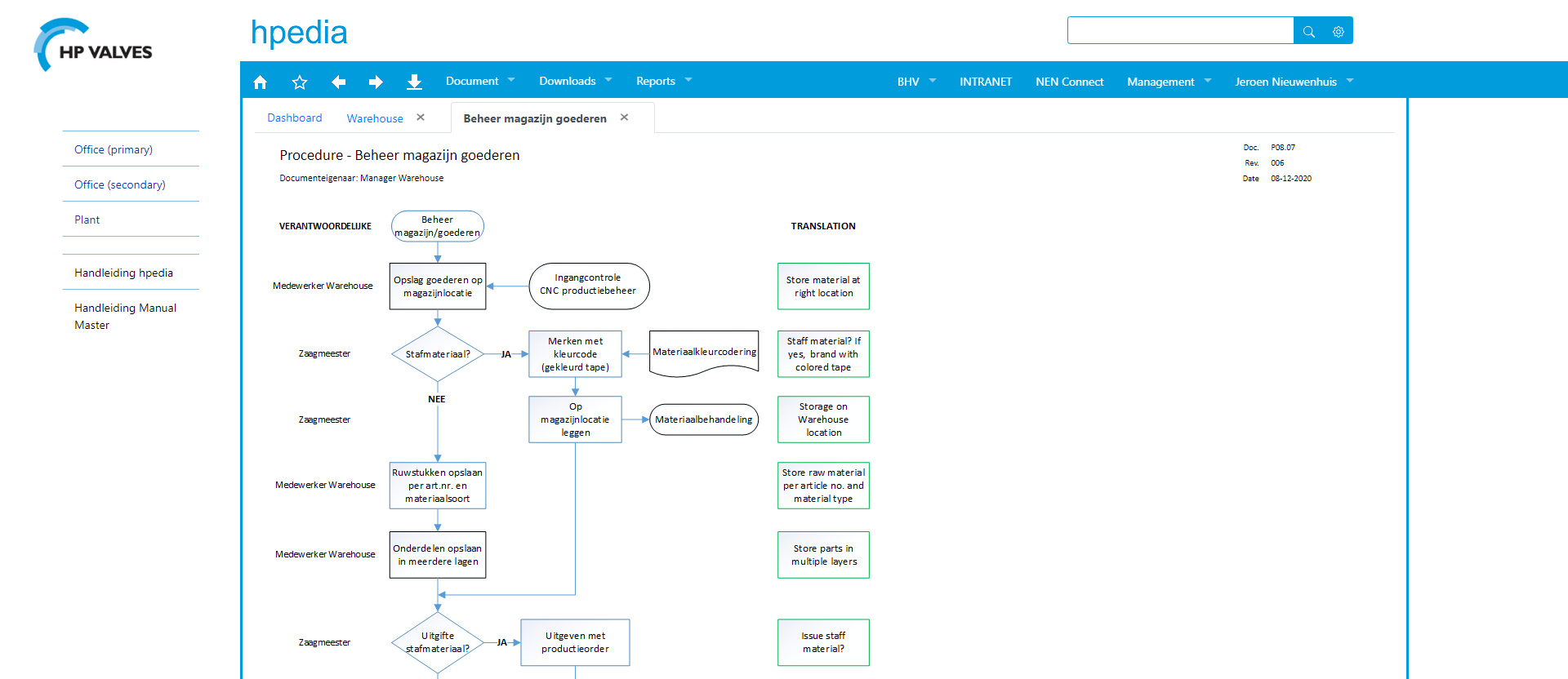
The risks identified by Jeroen are linked to parts of the operating processes. Jeroen has developed flowcharts for this and included English translations to help foreign employees who have difficulty understanding Dutch. (see fig. 3)
Risks are discussed with the department heads first, following which the agreed risk mitigation measures are recorded in a document or working agreement. ‘This ensures that the work floor can access clear information about the risks and risk mitigation measures. We keep our documents as concise and practical as possible. There is no point in producing a novel that nobody has time to read.’
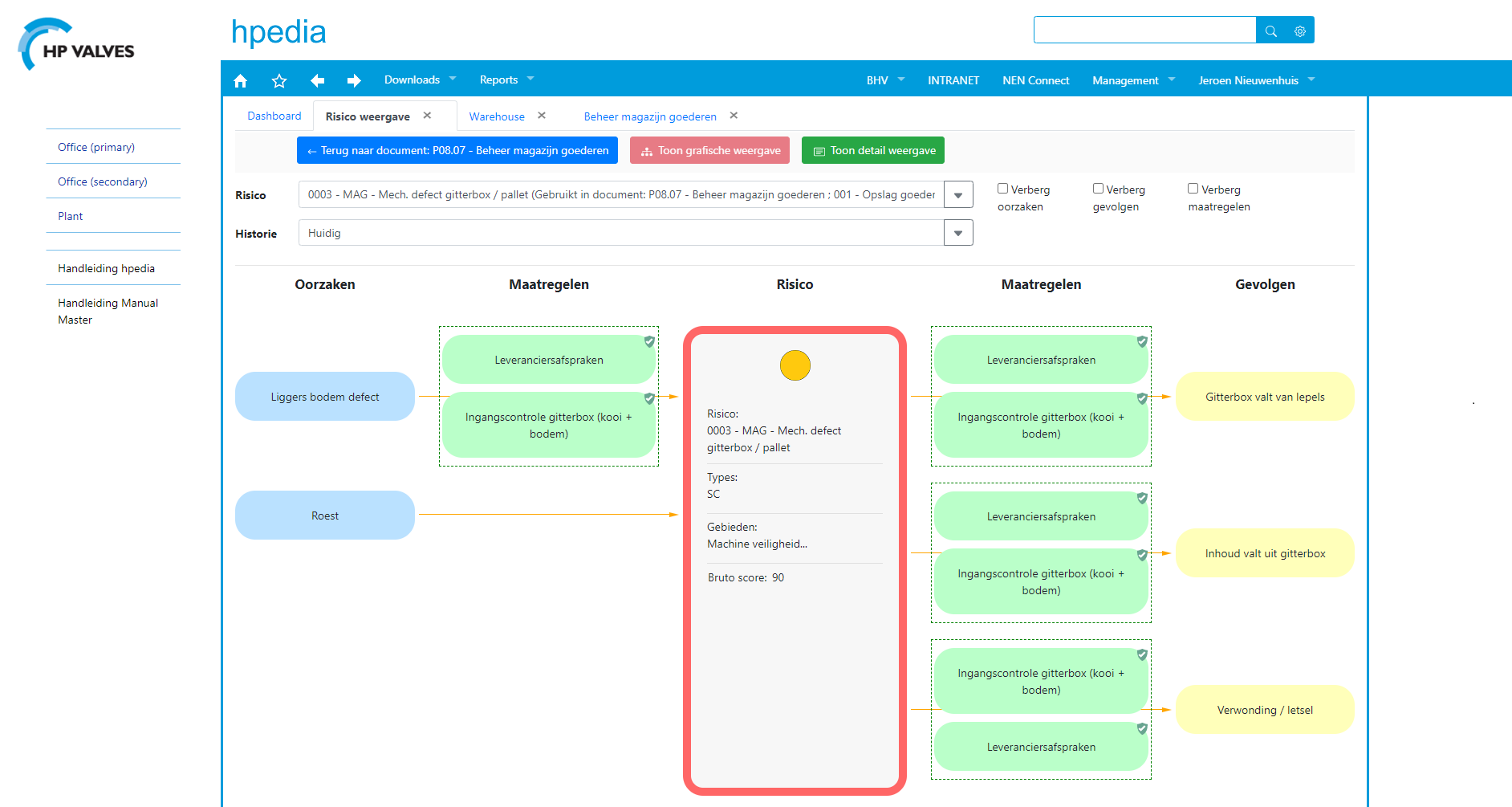
ManualMaster makes it possible to display an overview of risks, measures and documents within a process at the press of a button. (see fig. 4) This gives our employees immediate access to clear information about the process, the associated risks and what is expected of them.
Jeroen’s golden tip: ‘When you start using Risk Management, you should try to keep the set-up simple at first so that you can subsequently expand it in any direction. The consultants at ManualMaster can put you on the right track.’
Assigning risk scores to causes
Previously, HP Valves used an Excel worksheet to apply FMEA (Failure Mode and Effect Analysis) as a risk management tool. Any need for action was determined based on the worksheet. Jeroen: ‘The disadvantage of this set-up was that it could not be linked visually and directly to parts of the process.’
‘The FMEAs we used in the past were more detailed, but also much more time-consuming as a result. Detail is useful of course, but you have to make choices to maintain a good balance between the maturity of your organisation and the ultimate objective. If you go into too much detail too soon, you risk getting bogged down. So you end up with a system that looks great on paper but doesn’t work well in practice.’
‘The Risk Management module in ManualMaster works perfectly for us. Specifically in the area of the risk scores, we can further improve the level of detail in Risk Management. At the moment, the scores are linked to the risk itself. However, like our FMEA tool, the module can also be used to link risk scores to a cause rather than the risk itself.’
The latest version of ManualMaster WebForms, the module for creating intelligent web forms, also allows risk-cause analysis. Jeroen: ‘At a later stage, we may decide to build this capability into the Risk Management module.’
Using the WebForms module
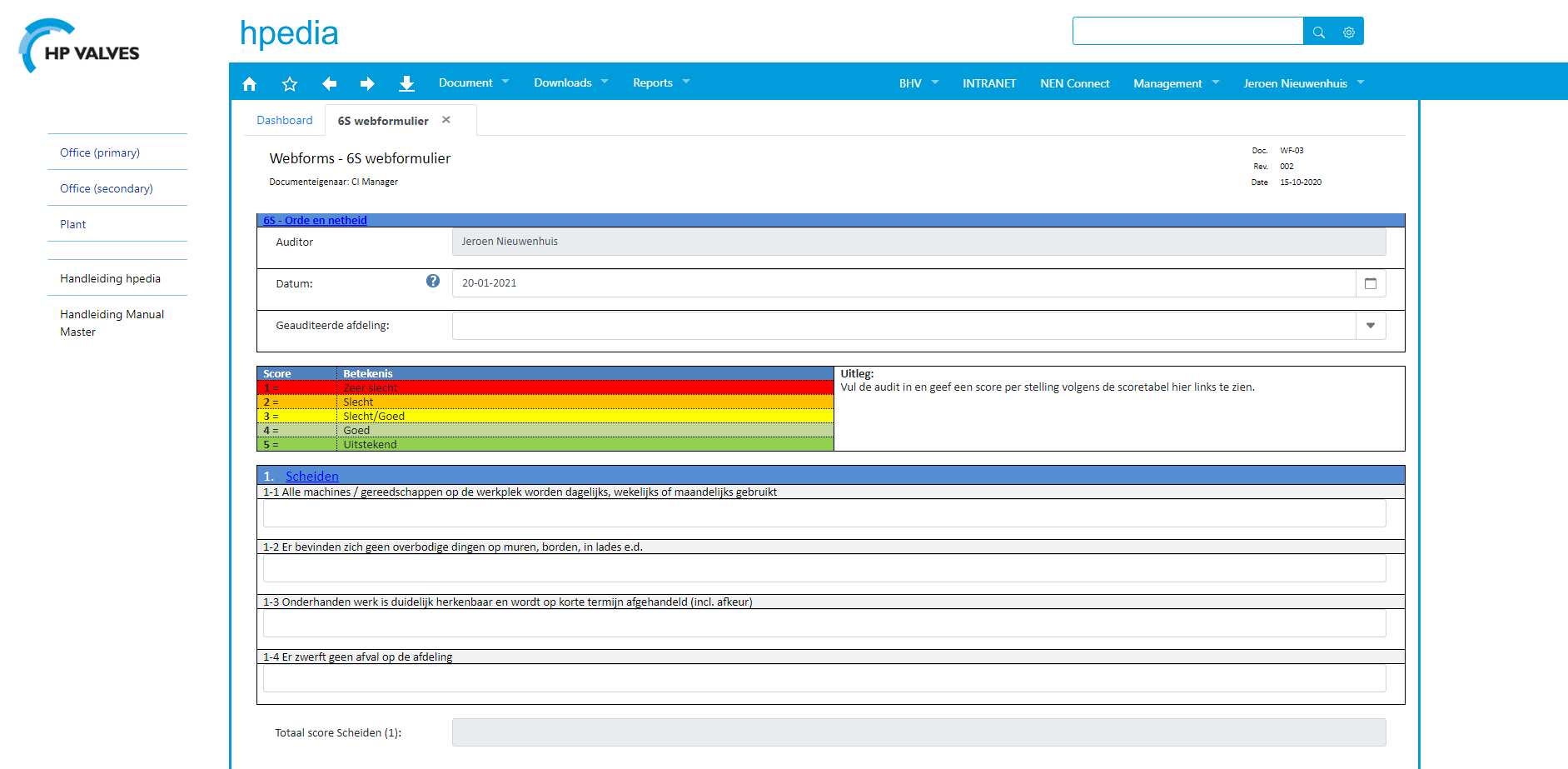
HP Valves uses WebForms to more effectively monitor actions linked to reports. Jeroen: ‘WebForms gives us a better overview. It makes it easier for us to follow up on actions. We also use WebForms to create 6S audit sheets for inspection rounds through the company. And we used WebForms to create our forms for accident registration.’
(see fig. 5)
Design, house style and layout
HP Valves has put a lot of time and effort into creating and styling the forms and flowcharts in ManualMaster. Jeroen: ‘Forms and documents need to look professional and the texts must be correct. (see fig. 6) That is important for the end users. A form or document that looks half-finished is not taken seriously.’
‘ManualMaster is extremely versatile. It offers you all the freedom you need when creating, structuring and designing things. As a result, our quality system Hpedia is both extremely accessible and highly intuitive. Very important in our view. The more intuitive a system is, the faster users will learn all the ins-and-outs and start using it effectively.’
‘The feedback from our users is positive. Employees know how to find the information they need quickly. As a result, the quality system is used intensively every day. All the stakeholders – from the work floor up to the MT, executive board and even our auditors – are really satisfied. They particularly like the system’s user-friendliness, intuitiveness and clarity. The extremely professional graphical presentation in Hpedia is also a big plus. Everybody in the organisation can access the system quickly and easily on the network. Access via a tablet or smartphone is not yet possible because our company Wi-Fi is not yet available to everybody.’
Why ManualMaster?
The employees at HP Valves were closely involved in the process that resulted in the purchase of ManualMaster. Jeroen: ‘The most important criteria for choosing the document management system were: a good search function, simplicity, low-threshold accessibility and expandability. The system’s user-friendliness and tools for personalising the layout and setting it up specifically for the different departments were the aspects that tipped the balance in the end.’
The user-friendliness and tools for personalising the layout and setting it up specifically for the different departments were the aspects that tipped the balance
Creating support
Because the users were listened to attentively during the procurement process, Hpedia was enthusiastically received by the employees at HP Valves. Jeroen: ‘Two essential questions had to be answered first: 1. What information do our colleagues need? 2. How would they like that information presented? We also set up a competition to find the best name for the system. That’s where Hpedia comes from. And finally, we made a presentation film available to increase awareness among our employees.’
Tip for future users
Jeroen: ‘Just start using the system – that’s the best advice I can give future users of ManualMaster. Start setting your system up and designing the screens. Starting is the main hurdle, then you just carry on one small step at a time. Don’t go into excessive detail initially. You can always change and improve things later on. Setting up a quality system with ManualMaster is certain to succeed because their consultants and account managers look at the task from the customer’s perspective. They also all have an in-depth knowledge of quality.’
More about HP Valves
by Ad Killian
About HP Valves
| Main activity | Producer of high-pressure valves and accessories |
| ManualMaster in use since | med 2020 |
| Modules used | Document Management, WebForms, Reporting, Risk Management |