Quincy de Hoog, Quality Control Manager: “Actions are organised and completed more quickly with WebForms”
Duchefa Farma imports, approves, repackages and distributes high-quality pharmaceutical ingredients and excipients for preparation in community pharmacies, hospital pharmacies and the pharmaceutical industry. Duchefa Biochemie, its sister company, sells raw materials and utensils and produces media for plant cell and tissue cultivation.
Duchefa Farma has a certified quality assurance system and is subject to periodic inspections of its GMP (Good Manufacturing Practice), GDP (Good Distribution Practice) and KNMP-NVZA (Statement Reliable Supplier), among others. In addition, Duchefa Pharma and Duchefa Biochemie have a ISO 9001:2015 certification.
Duchefa Farma and Duchefa Biochemie use the same format for quality management. ManualMaster plays a role in this quality management in the context of documentation and process management. The WebForms module digitises the registration and complaints system by using intelligent web forms. Other web forms are in the pipeline, such as a Supplier Assessment Form, Management of Change (MOC), Return Procedure, Satisfaction Surveys, Audits and Stock Management.
Correct documentation available at any time
Quincy de Hoog is Head of Quality Control Management at Duchefa. “Document Management ensures that everyone has access to the correct documentation at any time. Our registrations are also managed by the system. It’s difficult to imagine that we used to work with two quality manuals in different locations.”
“The most significant benefit of ManualMaster is that our people always have the necessary documents at hand. The system also gives you good insight into which documents need to be revised. ManualMaster ensures that our GMP branch is easily able to manage its three-yearly revision obligation. The system implements changes to documents quickly and ensures that the documents are authorised by the right people in the company. The software therefore has a considerable impact on Duchefa’s entire quality management system.”
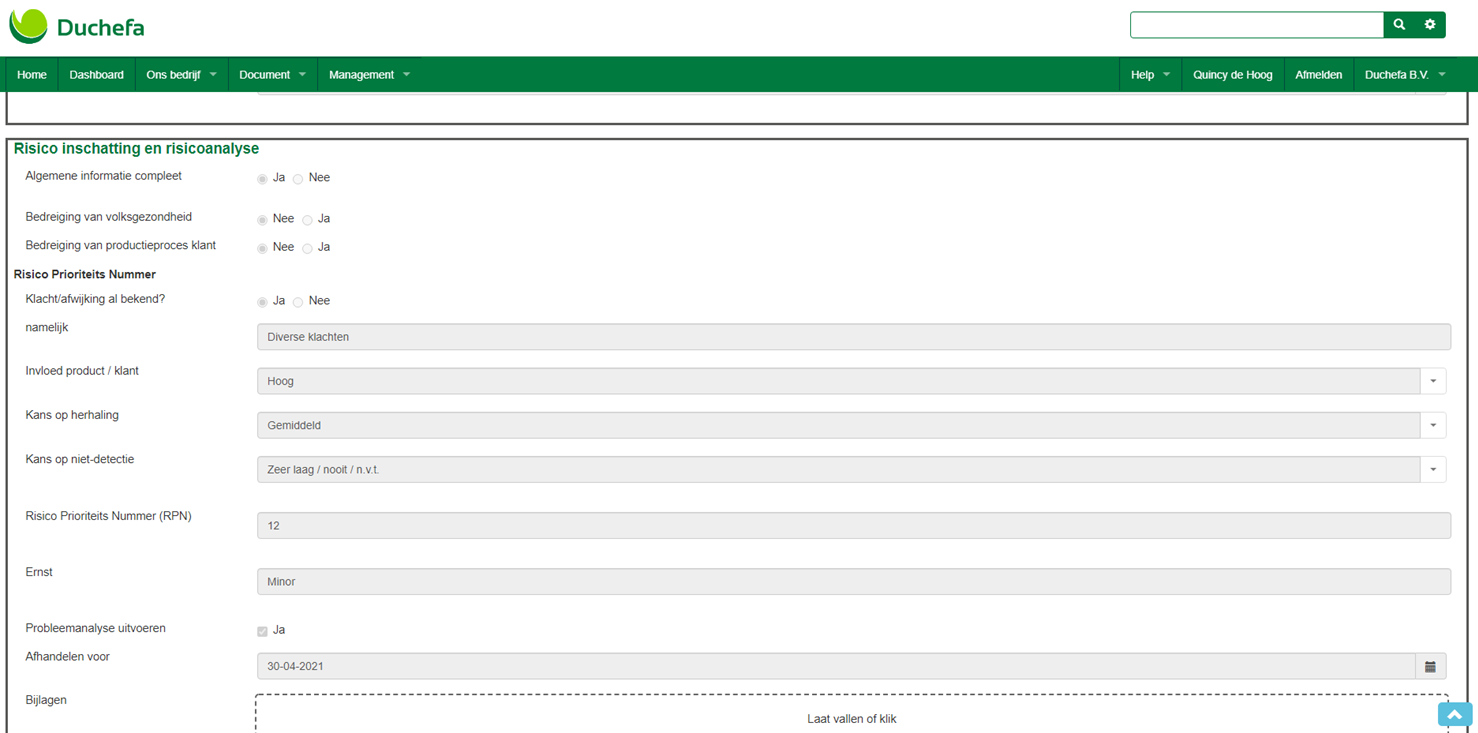
CAPAs linked to registration and complaint forms
Using the Plan-Do-Check-Act method, Duchefa Farma breathes life into the Knowledge Management System (KMS) with WebForms. For example, registration forms are compiled with WebForms and the forms for internal and external complaints have been merged into one web form and one integrated complaints system.
Quincy: “Working with a digital registration form was quite a step for Duchefa to take. The biggest challenge during implementation was getting employees to work with the forms and designing the forms to be dummy-proof. That has been achieved. The willingness to report has increased considerably since using the digital forms.”
“What I really like about the new complaints form is that an RPN (Risk Priority Number) score is automatically calculated for every complaint that is reported. Actions are defined and the form is processed based on the severity of the complaint. The form ensures that employees initiate a CAPA (Corrective And Preventive Actions) in the case of increased risk. In time, we could integrate this into the ManualMaster Risk Management functionality.”
Duchefa has not put the external complaint form online but it is available via the organisation. Quincy: “We chose this option because we want to stay in touch with our customers. We want to know whether it is really a complaint or something else.”
Advantages of working with WebForms
The necessary work had to be carried out in order to get the organisation to work with WebForms. “Much of what people filled in on the paper registration forms was barely legible. Obviously this is killing in a Good Manufacturing Practice (GMP) environment. It meant that the readability of the forms was a big issue. In addition, the paper-based complaints system we used previously often did not have enough room to describe the complaint clearly. This frequently resulted in complaints being written on the side or back of the form, or complaints being submitted with various attachments. A very undesirable development. In addition, forms took a relatively long time to process and in some cases even disappeared without trace.”
“The big advantage of WebForms is that we can type instead of writing. Now that we are working with the digital web forms, the speed with which cases are resolved is significantly faster. Actions are organised and completed more quickly.”
Registration forms via tablet or mobile phone
Duchefa compiled a wish list before using WebForms. Quincy: “We first looked at which web forms could be completed from a computer. In addition, there are registration forms that you want to use during production or when you are in the warehouse. You can’t just take a computer along. This requires tablets or mobile phones. They will be used in phase 2.” Duchefa is also thinking about implementing MoC (Management of Chance) forms that employees can use to make process improvement proposals.
At the moment, the quality management department is working on digitising the returns procedure. Quincy: “This involves warehouse employees registering a return in ManualMaster, and receiving and checking the return.” He also wants to use tablets and mobile phones for this. The design of a supplier evaluation form, an internal audit form and a digital satisfaction survey for Duchefa employees are also in the pipeline.
Collecting and presenting data in Reports
The data collected in the web forms will enable Duchefa to compile Reports for management reviews. Quincy: “It is also handy that you can discuss critical issues in the monthly meeting using Reports. You can immediately see what the status of an issue is, who is working on it and whether or not any action has been taken.”
Validation and satisfied auditors
In 2018, the document management system was validated internally by Duchefa. Tests were carried out and a report, complete with guidelines and annexes, was compiled on the basis of these tests. Quincy: “This is sufficient for Duchefa’s purposes, especially bearing in mind that ManualMaster itself tests its products ad infinitum and makes sure the system works.”
“For every new release by ManualMaster, we look at the list of release notes to see if the changes will have an impact on the validation we performed and if it is necessary to perform the validation again. We do this on the basis of an impact and risk assessment of the changes. It makes us reluctant to update the system. We implement updates in major version changes, which means we have to review a lot of changes at one time.”
The Quality Control Manager finds the validation of the web forms more challenging. “As an organisation, you have to be able to demonstrate that a web form works in practice and how authorisation is arranged so that it is clear who can do what and when. Plus the retrievability must be well organised. To a large extent, this is covered by the audit trail that is integrated into WebForms.
Our auditors are satisfied. What is convenient about ManualMaster is that we can make our procedures clear by displaying the system on a large screen during customer audits. We get positive reactions and our ISO auditor is also happy about the digitisation of our processes and workflows.”
Motivating users
All Duchefa employees use ManualMaster. Quincy: “They like the fact that they can see exactly what needs to be done on their dashboard. We have extensively trained our employees in the use of WebForms. The course was very basic. It explained what a web form is, what the fields mean and how the process works. It was actually nothing more than a digital A4 sheet. This has created awareness. The training was more in-depth for those working with the complaints system where the problem analyses are carried out. A helpline was set up for those employees who could not work it out immediately.”
The importance of a good quality plan
Regulations and standards are increasing and are becoming. This means plenty of Knowledge Management System (KMS) challenges for the Quality Control Manager. Quincy: “You need a system that is flexible enough to cope with all these changes. Otherwise, you will not survive as an organisation. It is also a challenge to keep quality management knowhow within the company, so that if a manager leaves, his/her replacement can easily become familiar with the work. For this reason we are preparing a procedure for the creation of web forms.”
“For ManualMaster, it means keeping up to date of new regulations and customer requirements. But it’s clear we can trust ManualMaster to do this when I see what it does in the field of validation and risk management.”
Future users are advised by Quincy: “Make sure you have a good plan before you start. Paper documents cannot just be indiscriminately put into a system and digitised. It will be a mess, which you only realise years later. The same applies to WebForms. You cannot convert a paper form into a digital form on a one-to-one basis. That does not work. If you digitalise, you have to be very conscious about how you want to do it and the desired outcome.”
A very approachable organisation
“If you want quick answers to tricky questions, the people at ManualMaster can help. We had a consultant on site for the implementation of WebForms four times, which is more effective than working remotely. We found consultant/trainer Barbara de Ruiter very pleasant to work with, and she knows what she is talking about and how things work in other companies. If she could not answer a question, she immediately contacted a colleague to find a solution. She helped us a lot with the implementation of the system.”
“ManualMaster is an organisation that is very approachable. You get a quick and easy answer if you run into a problem or don’t know something. It projects a professional image and, most importantly, the system works.”
by Ad Killian
together with Ronald de Bruijne / ManualMaster
About Duchefa Farma
| Main activity | Distributor of high-quality active pharmaceutical ingredients and producer of media for plant cell and tissue cultivation |
| Uses ManualMaster since | 2015 |
| Uses | Document management – WebForms |